Non-interventional study to investigate the efficacy and safety of Tegaderm™ Matrix in the treatment of patients with therapy-refractory chronic wounds
Maren Weindorf, Andreas Körber, Joachim Klode, and Joachim Dissemond
Clinic and Polyclinic for Dermatology, Venereology and Allergology, Essen University Hospital
JDDG; 2012 • 10:412–420
Submitted: 21.7.2011 | Assumed: 9/3/2011
Keywords
- Tegaderm™ Matrix
- chronic wounds
- leg ulcers
- pressure ulcers
- diabetic foot syndrome
Summary Background:
Despite causal treatment, many patients with chronic wounds are refractory to treatment. Products such as Tegaderm™ Matrix were developed especially for these patients in order to reactivate the wound healing process by actively influencing the wound environment.
Patients and methods: In this prospective application study, a total of 314 patients with treatment-refractory chronic wounds of various origins were evaluated. In addition to the reduction in wound area and healing rate, adverse events were also documented.
Results: The wounds lasted an average of 10 months and had an average size of 17.3 cm2 (median 6.3 cm2 ) at the initial visit. In the course of treatment, the wound size reduced to 13.0 cm2 (median 3.5 cm2 ) and was 9.3 cm2 (median 0.9 cm2 ) at the end of the study. Based on the criteria of the European Wound Healing Society for clinical studies, a reduction of at least 50% in the wound area is considered a parameter for successful treatment. In this study, 72.9% of the patients achieved a wound area reduction of at least 50% in their previously treatment-refractory wounds. Only 4.7% of patients experienced a potentially treatment-related adverse event such as a burning sensation.
Conclusions: Our results show that Tegaderm™ Matrix, integrated into a causal therapy, is a well-tolerated wound dressing in the majority of patients with previously therapy-refractory chronic wounds, which can support a reduction in the wound area through to healing.
Keywords
- Tegaderm™ Matrix
- chronic wounds
- leg ulcers
- pressure ulcers
- diabetic foot syndrome
Summary
Background: Despite a variety of therapeutic approaches, many patients with chronic wounds remain refractory to treatment. Products such as Tegaderm™ Matrix were developed especially for such patients to alter the wound environment and reactivate the stagnant wound healing process.
Patients and Methods: In this prospective post-authorization observational product study, a total of 314 patients with therapy-refractory chronic wounds of various origins were evaluated. Beside to the wound area reduction and healing rate, the occurrence of adverse events was documented.
Results: On average the wounds were 10 months old. The average wound size was 17.3 cm2 (median 6.3 cm2 ) at the initial visit. In the course of treatment the wound size decreased to 13.0 cm2 (median 3.5 cm2 ) and was finally reduced to 9.3 cm2 (median 0.9 cm2 ) at end of the study. Taking the criteria of the European Wound Management Association for improving the quality of clinical studies into consideration, a wound size reduction of at least 50 % is the parameter for successful treatment of chronic wounds. This study demonstrated a wound size reduction of at least 50 % for 72.9 % of the patients with therapy-refractory chronic wounds when treated with Tegaderm™ Matrix. The safety profile was evaluated; only 4.7 % of the patients experienced a treatment-related adverse event such as a burning sensation.
Conclusions: The results of the study demonstrate that Tegaderm™ Matrix along with treatment of underlying causes is a well tolerated wound dressing promoting wound size reduction up to healing for the majority of patients with previously therapy-refractory chronic wounds.
Introduction
The treatment of chronic wounds that are refractory to therapy represents an interdisciplinary challenge in daily practice. Since the pathophysiologically relevant factors underlying impaired wound healing can be very diverse, a differential diagnosis should always be carried out before initiating specific therapy Clarification of the causes of the wound healing disturbances [1, 2].
Despite causal therapy, the wounds in some patients will not heal. One reason could be the factors in the wound environment that have been increasingly described in recent years and that influence a disrupted wound healing cascade. The focus here is, among other things, on the balance between matrix metalloproteinases (MMP) and their physiological counterparts, the tissue inhibitors of metalloproteinases (TIMP) [3–6]. Wound therapeutics that are intended to actively intervene in this process are therefore also referred to as active wound dressings or wound starters. One of these new active wound dressings is Tegaderm™ Matrix.
After the effectiveness of Tegaderm™ Matrix had already been demonstrated in several in-vitro and initial clinical studies, the aim of our study was to promote wound healing and tolerability of Tegaderm™ Matrix in a larger patient population with chronic wounds that were previously refractory to therapy to be examined under real conditions of clinical practice.
Patients and methods
All patients with chronic wounds that had been present for at least the 2nd months and showed delayed healing despite adequate wound therapy. In addition, if possible, the underlying cause(s) should have been treated, which should then not be changed within the framework of the study but should be continued. Exclusion criteria were the presence of necrosis, clinically infected wounds and known contact sensitization to components of the wound dressing used.
Preparation and application
Tegaderm™ Matrix is a sterile wound dressing impregnated with an ointment made from polyhydrated ionogens (PHI). In addition to citrate buffer, the PHI also contains a synthetic mixture of trace elements such as calcium, potassium, rubidium and zinc on a cellulose acetate carrier. Under the influence of heat and moisture, polyethylene glycol allows the formulation to be released into the wound bed.
Wound size decreased to 13.0 cm2 (median 3.5 cm2 ) and was finally reduced to 9.3 cm2 (median 0.9 cm2 ) at end of the study. Taking the criteria of the European Wound Management Association for improving the quality of clinical studies into consideration, a wound size reduction of at least 50 % is the parameter for successful treatment of chronic wounds. This study demonstrated a wound size reduction of at least 50 % for 72.9 % of the patients with therapy-refractory chronic wounds when treated with Tegaderm™ Matrix. The safety profile was evaluated; only 4.7 % of the patients experienced a treatment-related adverse event such as a burning sensation. In the patients, Tegaderm™ Matrix was placed on the previously cleaned wounds as the primary dressing. The attending physician was able to choose the secondary dressing according to the expected wound exudation. Only the combined use with an alginate or hydrocolloid dressing was not recommended. Dressings were changed daily for the first 2 weeks and every 2-3 days thereafter.
Observation period and timing
The noninterventional study in the sense of an application observation was prospective and non-controlled. It started in February 2009 and ended in May 2010. The results of the medical examinations were released documented at 3 different times. The dates for the doctor-patient contacts were determined individually; however, the documentation times were fixed. The individual observation period was a maximum of 12 weeks. Among other things, the size of the wound and information on the wound bed and surroundings were documented.
Participating centers
The study was carried out in Germany with 93 physicians from the fields of dermatology (50.5%), surgery (24.7%) and general medicine (20.4%) throughout Germany.
Statistical evaluation
The documentation forms were sent to the Institute Dr. Schauerte (Oberhaching) forwarded and recorded in a database.
Before starting the statistical analysis, a validation and analysis plan was created. All documented illnesses and unexpected events were coded according to MedDRA (version 13.1). The calculation of the wound sizes, taking into account the sizes given by the treating therapists, was carried out according to the specifications of Goldman
and Salcido [7]. The statistical evaluation included all observation criteria and was carried out according to descriptive and exploratory statistical methods. In addition to specifying the total number and the number of missing values, these included specifying the mean value, median, largest and smallest value or frequency information, depending on the scale level. The analysis was carried out using the SAS statistics program package (Version 9.2, SAS Institute Inc., Cary, NC, USA).
Results
Total patient population
A total of 364 patients were included in the study. These were 275 patients with leg ulcers (75.5%), 29 patients with diabetic foot syndrome (8.0%), 13 bedsores (3.6%) and 47 patients with other types of wounds such as burns, Radiodermic, post-operative or post-traumatic wounds (12.9%). The wounds had existed for an average of 10 months. There were 166 patients (45.6%) men and 198 women (54.4%) with an average age of 68 ± 15 years. The mean duration of treatment with Tegaderm™ Matrix in the clinical study was 9.4 weeks.
To study the effectiveness of Tegaderm™ Matrix, The datasets were taken into account in which the wound size information was complete for all visits and treatment with Tegaderm™ Matrix for at least three weeks was traceable. A total of 314 datasets met these criteria and were finally evaluated for the healing rate.
The average wound size at the initial visit was 17.3 cm2 (median 6.3 cm2 ). The wound area decreased to 13.0 cm2 (median 3.5 cm2 ) at the follow-up visit and further reduced to 9.3 cm2 (median 0.9 cm2 ) at the final visit (Table 1, Figure 1). For the healing rate, this means that in 40.8% of the patients a wound area reduction of between 75-100% could be achieved and 17.8% of the patients showed a healing rate of between 50-75%.
The wound healed completely in a total of 45 patients (14.3%) (Table 2). Thus, therapy with Tegaderm™ Matrix achieved at least a 50% improvement in 72.9% of the patients.
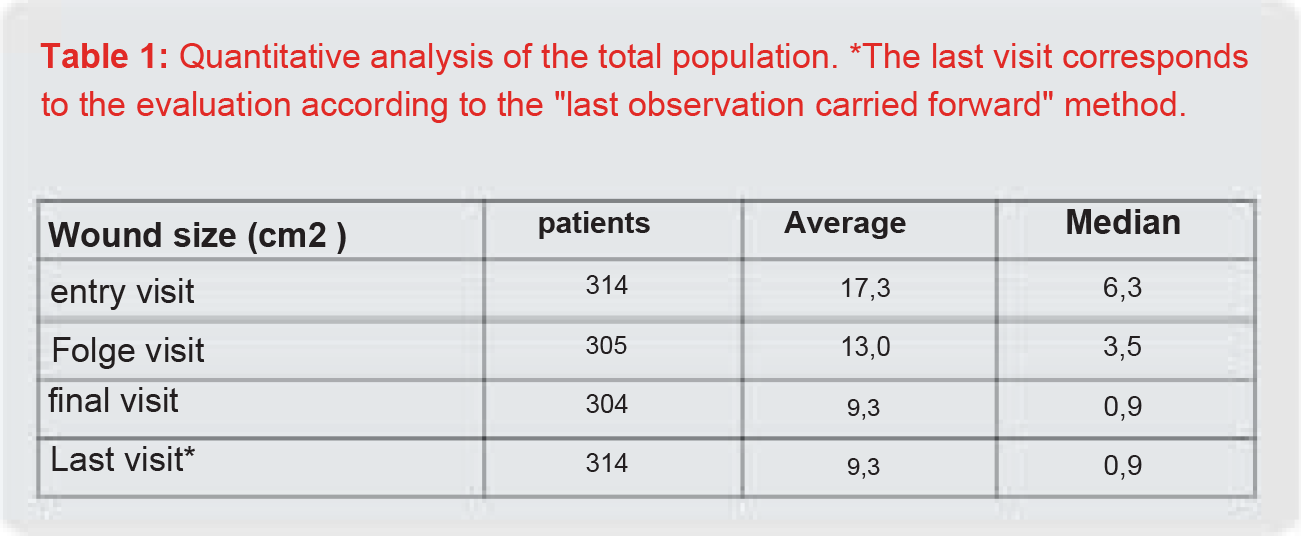
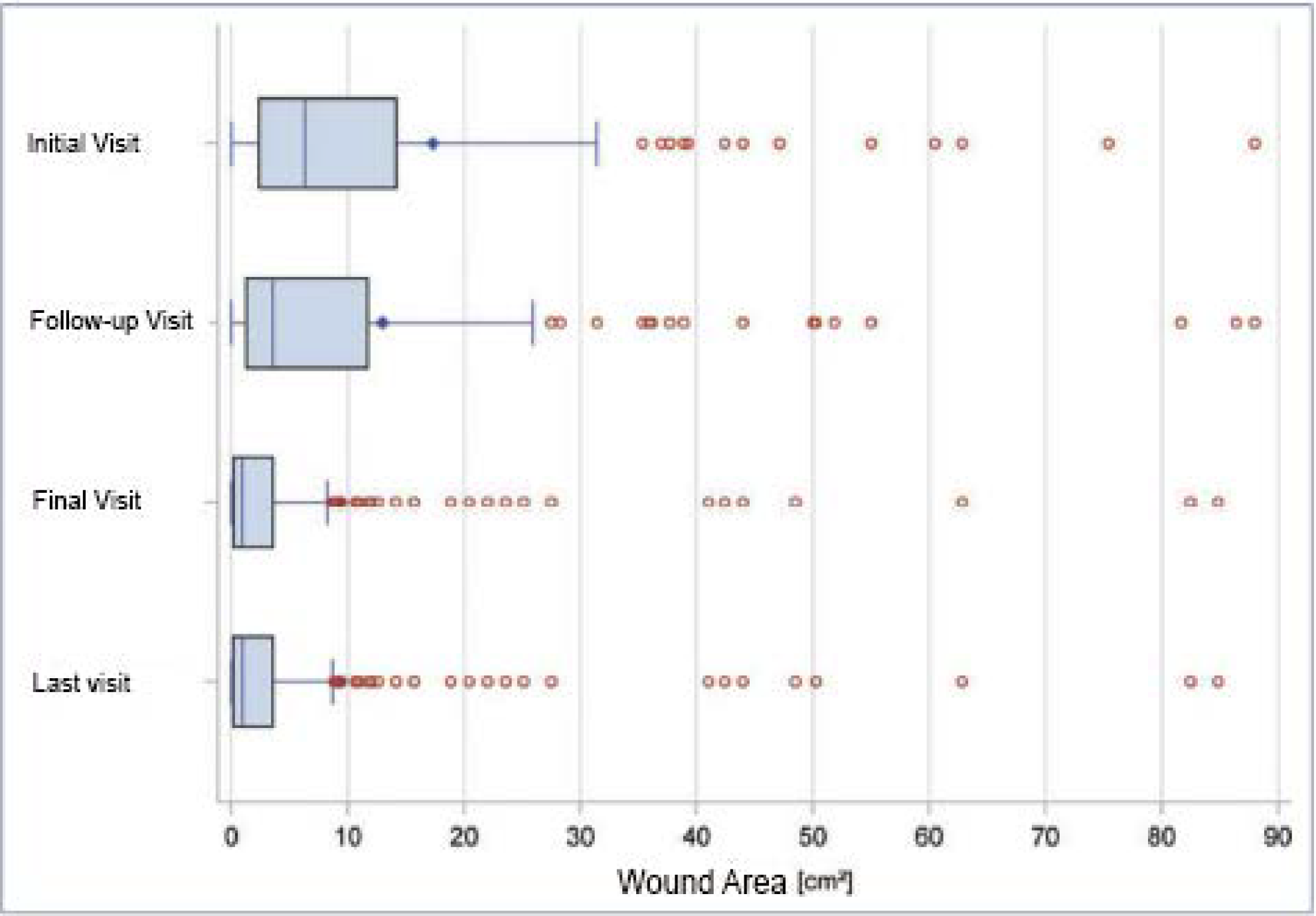
Figure 1: Reduction of wound area in the total population. Some very large wounds of up to 475 cm2 were included in the study. For better visualization of the effects, the boxplot graphic therefore only shows wounds with a maximum size of 90 cm2 . *The last visit corresponds to the evaluation according to the “last observation carried forward” method.
Reduction of the area of chronic, previously therapy-refractory wounds. The composition of the wound bed and the wound border was also assessed during treatment. In addition to a reduction in the fibrin coating and an increase in granulation and epithelial tissue, an improvement in the wound edge was documented. The appearance of macerated, reddened ten and/or edematous wound edges shifts significantly in the direction of intact wound edges (Figure 2a-d).
In addition to the efficacy profile, the safety and tolerability profile was also evaluated. For this purpose, the data of all 364 patients were taken into account. Overall, 86.5% of patients completed treatment as planned. An interruption of therapy he followed in 1.1% of patients and early discontinuation in 12.1%.
The most frequently cited reason for discontinuing therapy was an increase in wound pain (3.6%), but also reasons such as “loss to follow-up” or referrals to hospitals that were not related to the product. Adverse events (AEs) were documented for a total of 39 patients. A causal relationship with the use of Tegaderm™ Matrix was stated for 17 patients and a possible relationship was suspected for a further 11 patients.
A connection could be ruled out in 8 patients; No further information was available for 4 other patients. The AEs were coded according to MedDRA and divided into clusters. In 11 cases, a burning sensation in the wound and in 10 other cases the occurrence of wound pain after application of the wound dressing was documented. In addition to this main cluster, an
increase in wound size occurred in 5 patients, which was reported as a possibly therapy-related AE. The other events are distributed among the clusters “Changes to the wound surface or the area directly surrounding the wound” (11 patients.)
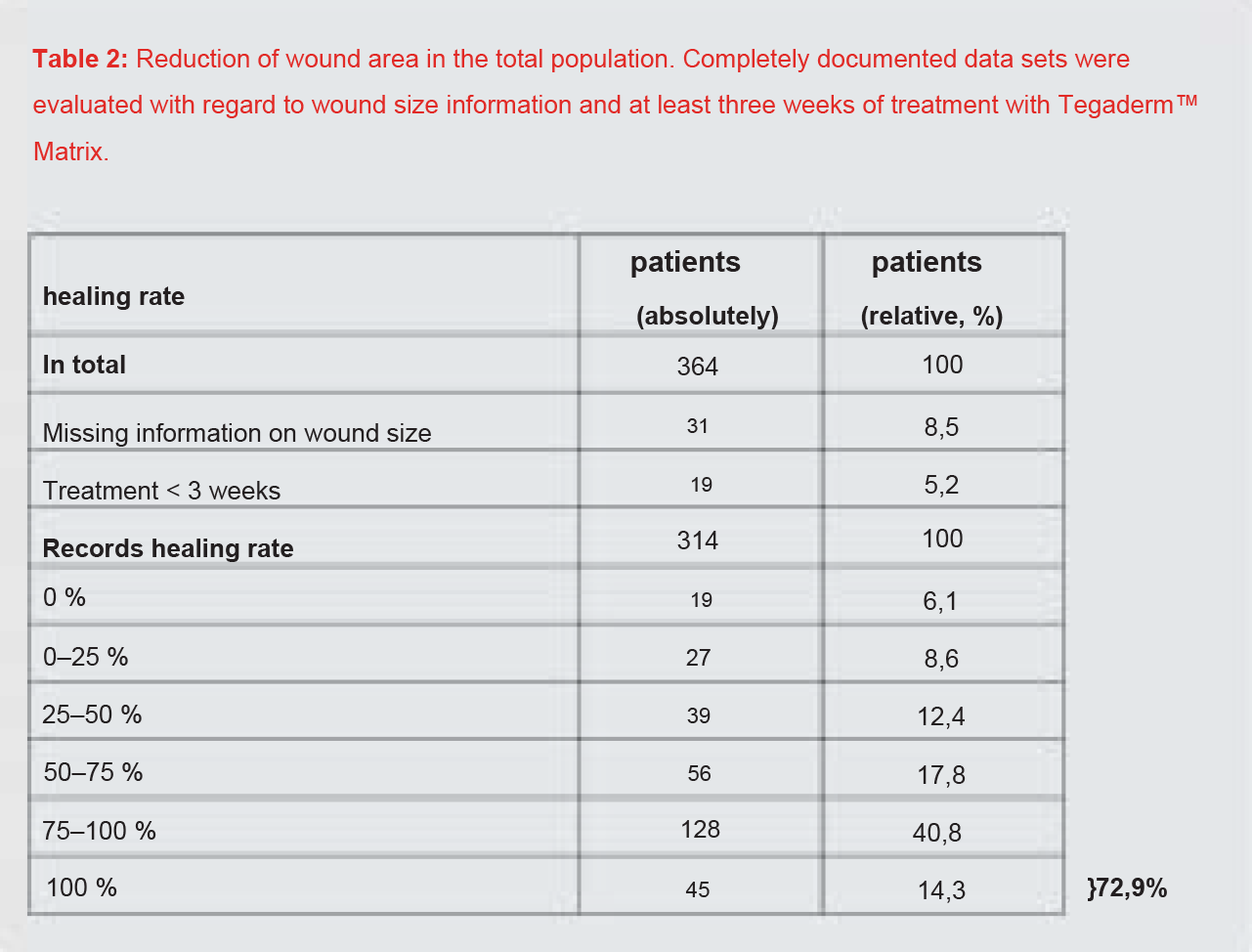
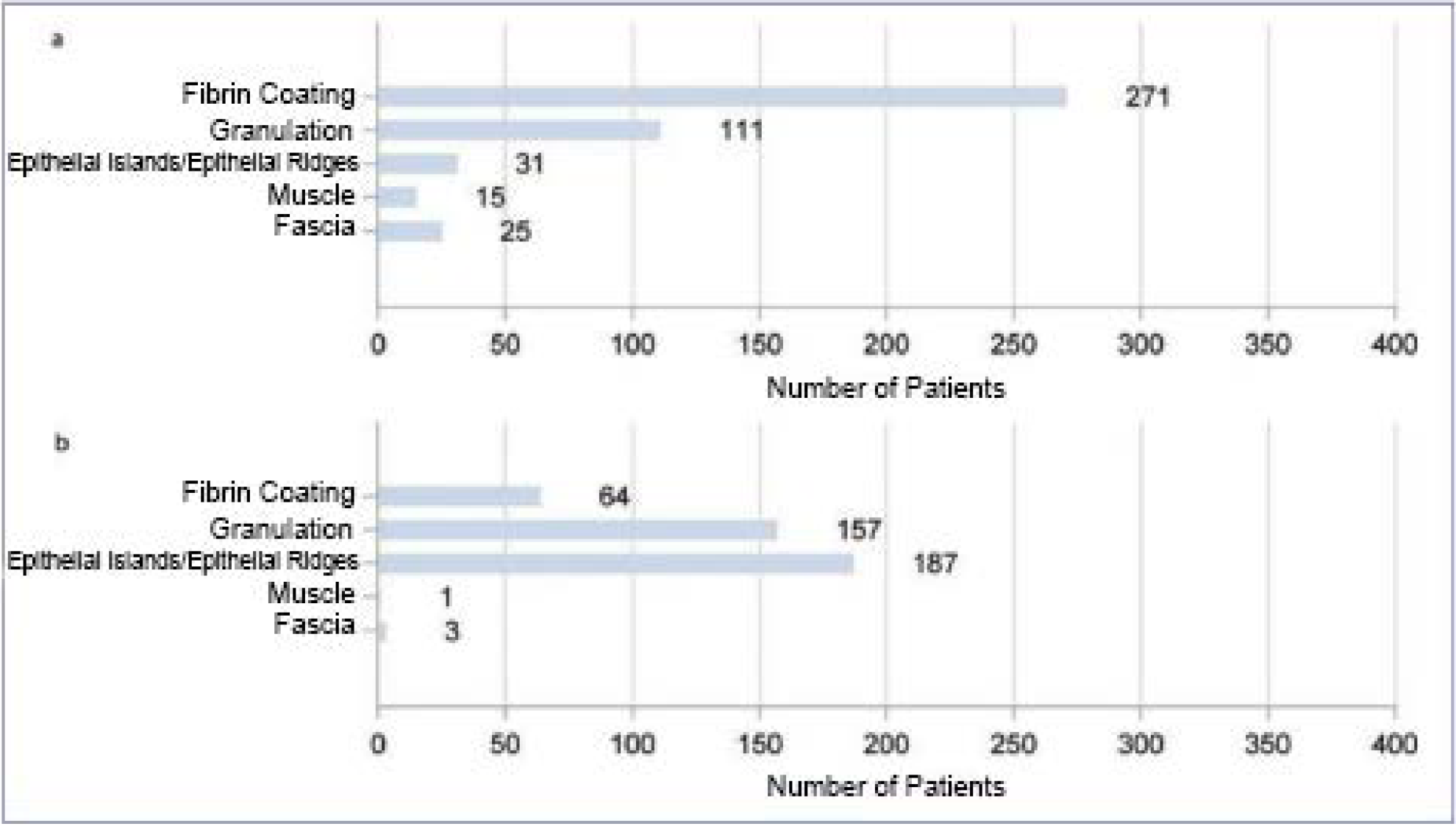
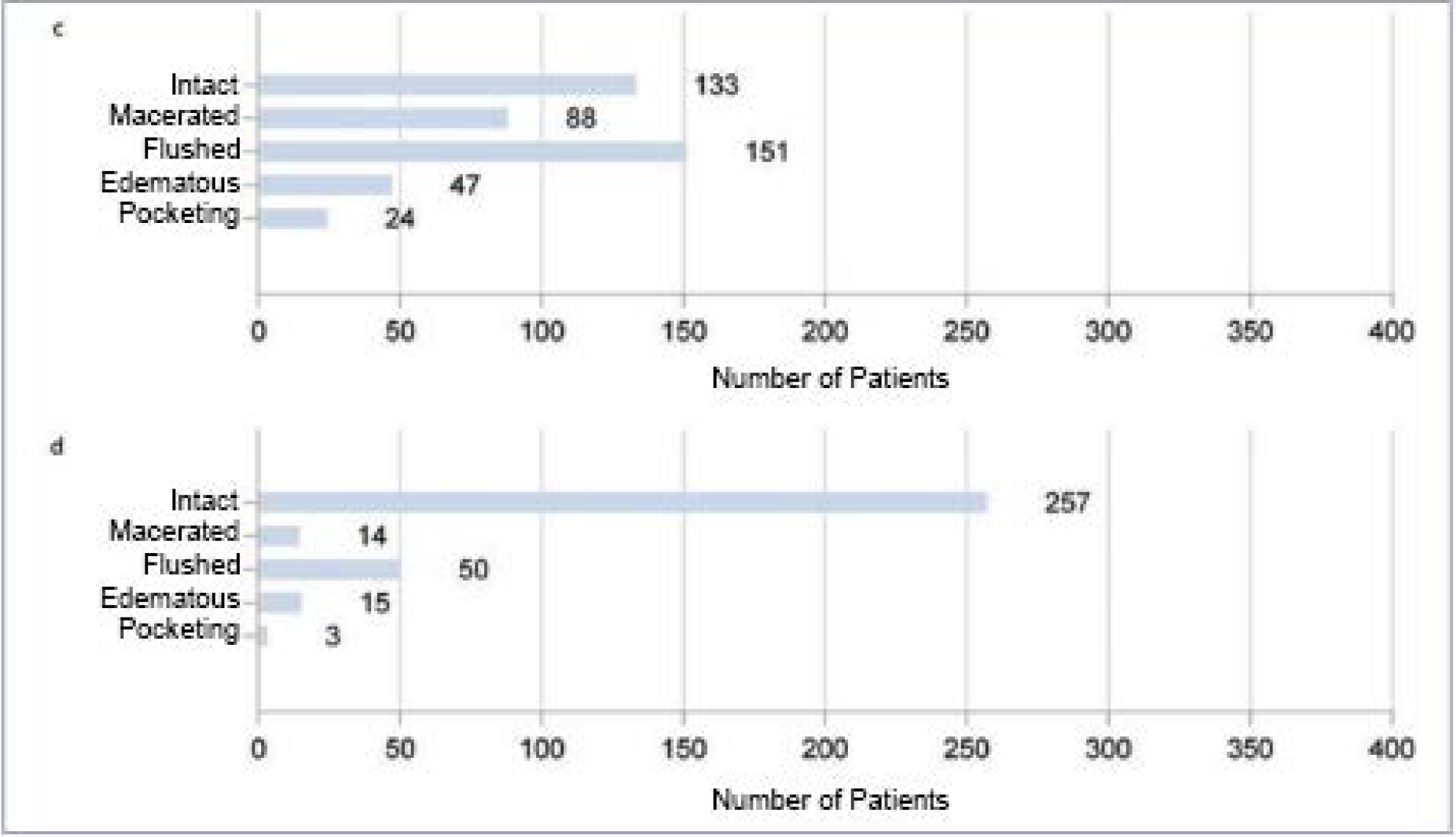
Figure 2: Analysis of the total population with regard to wound base (a and b) and wound edge (c and d) over the course of therapy. Figures a and c show the respective initial findings before therapy, figures b and d show the findings after treatment has been completed. Multiple entries were possible.
8 causal, 3 possibly causal), incipient wound infection (2 patients, possibly causal) and documented allergic contact dermatitis in one case (1 patient, causal). Thus, 95.3% of the patients showed no AEs causally related to Tegaderm™ Matrix. The tolerability profile of Tegaderm™ Matrix can therefore be rated as good.
The further description of the results is differentiated according to the genesis of the wounds as subgroup analyses.
Ulcus cruris
The entity ulcus cruris formed the largest subgroup of this study. After checking the data sets for completeness, the data sets of 231 patients were evaluated. Of these Patients were 135 female (58.4%) and 96 male (41.6%). The average patient age was 70 ± 13 years. With regard to the genesis, a venous leg ulcer was described in 173 patients (74.9%), a mixed leg leg ulcer in 50 patients (21.6%) and a leg ulcer in 5 patients (2.2%). In 3 patients (1.3%) there was no information on the genesis.
The duration of the wounds was between 2 and 200 months and averaged 12 months. 104 (45%) patients reported recurrent ulcerations and 176 (76.2%) patients reported pain. Pain averaged 4.3 points on a visual analogue scale (VAS). The duration of treatment with Tegaderm™ Matrix was average at 9.5 weeks. For the calculation of the healing rate, the ulcers were evaluated for which complete wound size information was available for all visits.
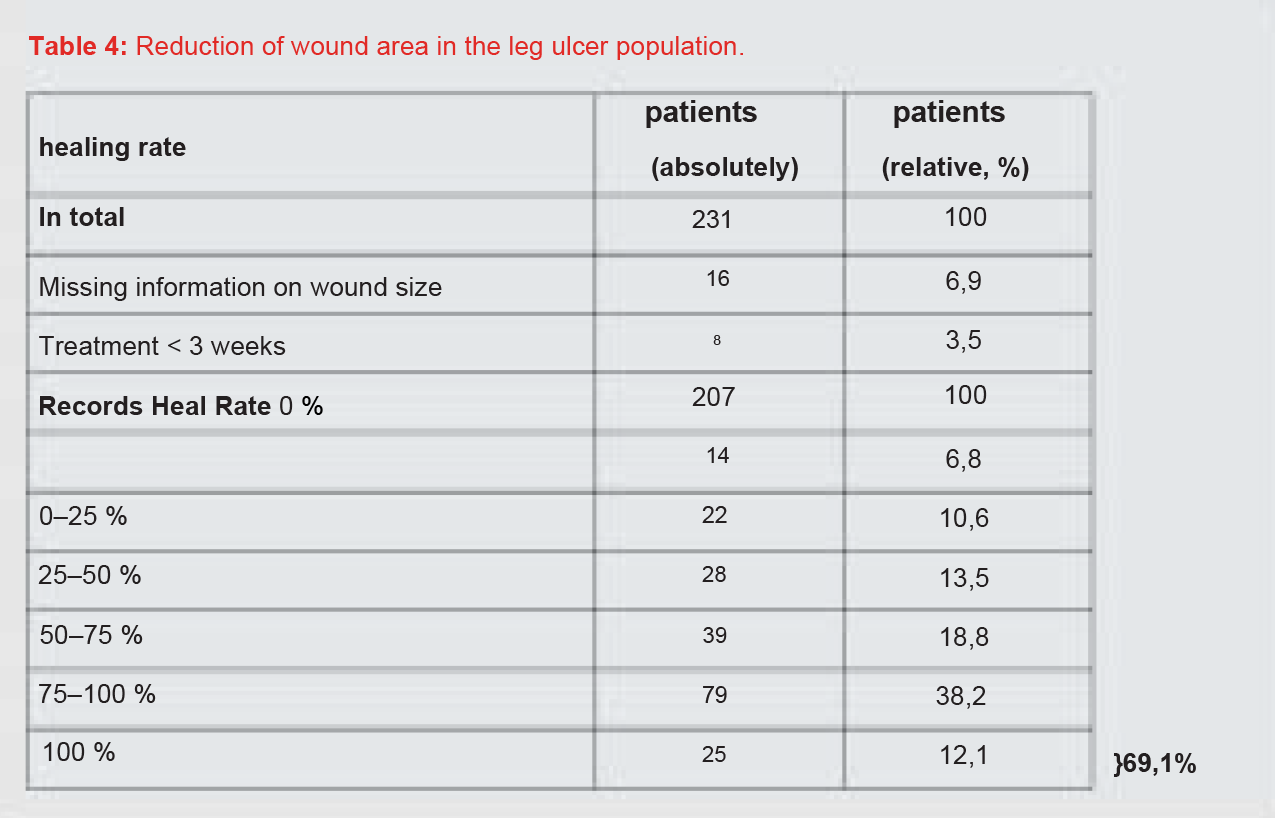
This was true for 207 datasets. At the initial visit, the patients’ wounds were at least 0.1 cm2 and at most 475.2 cm2 in size. The average wound size was 19.3 cm2 (median 7.1 cm2 ). The mean wound size decreased to 14.6 cm2 (median 4.7 cm2 ) at the follow-up visit and to 11.7 cm2 (median 1.6 cm2 ) at the final visit (Table 3, Figure 3).
After completion of the treatment, 38.2% of the patients showed a wound area reduction between 75% and 100% and 18.8% of the patients a wound area reduction between 50% and 75%. Overall, 12.1% of the wounds healed completely (Table 4). Summarizing the healing rates, 69.1% of the patients achieved at least a 50% reduction in wound area.
Decubitus
9 patients with decubitus were evaluated, of whom 6 were women and 3 were men aged between 64 and 89 years (mean 79 years). The wounds had existed for an average of 8 months and were in the gluteal area in 6 patients.
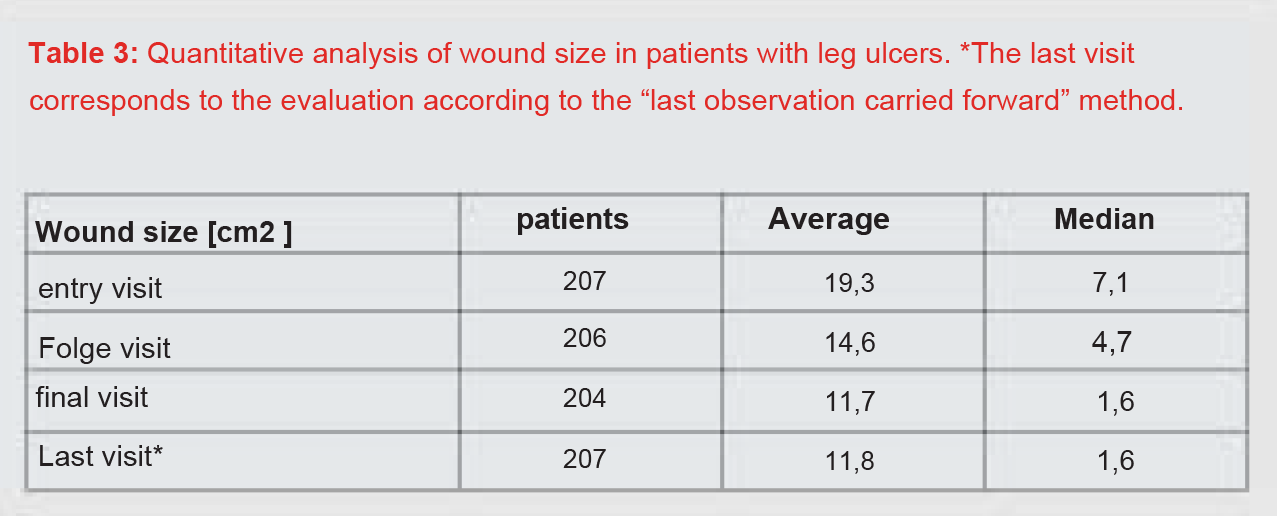
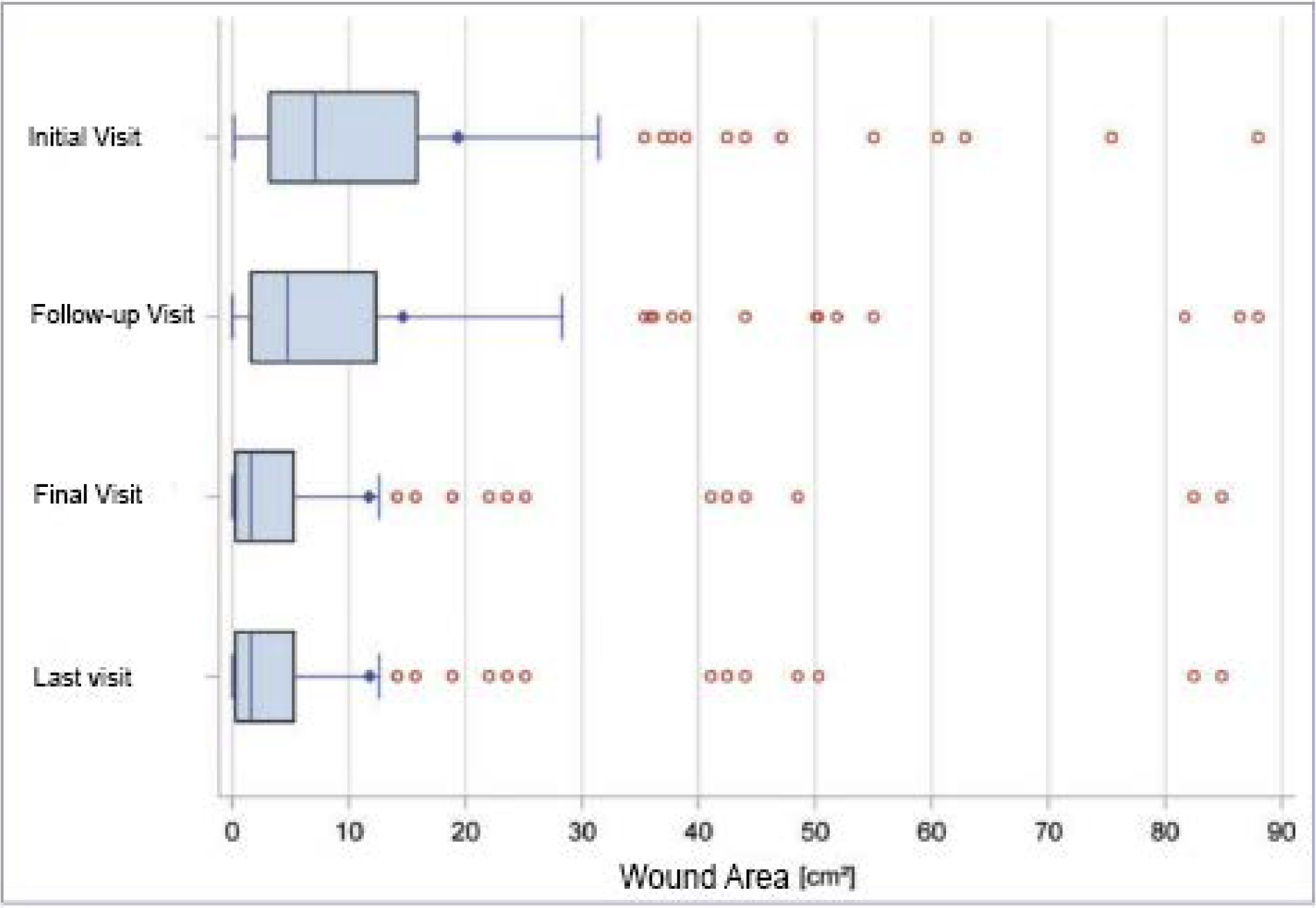
Figure 3: Reduction of wound area in the leg ulcer population. *The last visit corresponds to the evaluation according to the “last observation carried
for ward” method.
At the initial visit, wound areas were reported to range from 2.4 to 39.3 cm2 with an average size of 14.6 cm2 (median 8.1 cm2). The average wound area was 8.8 cm2 (median 5.5 cm2 ) at the follow-up visit and 3.6 cm2 (median 1.4 cm2 ) at the final visit. At least 50% Reduction in wound area was shown for 5 of 8 patients (62.5%). One wound healed completely within the specified study period.
Diabetic foot syndrome
Of the 22 patients with a diabetic foot syndrome, 17 patients were male (77.3%) and 5 female (22.7%). The ages ranged from 41 to 83 years and averaged 66 years. With regard to the Wagner stages, stage I was found in 7 patients (31.8%), stage II in 9 patients (40.9%), stage III in 4 patients (18.2%) and stage IV in 2 patients reported (9.1%).
The wounds had existed for 2 to 60 months, with an average of 11 months. Localized in 2 patients on the lower legs and in 1 patient on the back. 7 patients had decubitus in stage III, 2 patients had decubitus in stage II according to Seiler.
The wounds were recurrent in 7 patients. From 6 patients pain was reported with an average severity of 3.9
VAS points. The average duration of treatment with Tegaderm™ Matrix was 11 weeks. The data sets of
8 patients could be evaluated for the healing rate.
Based on the medical history, 8 patients (36.4%) had a recurrence; Nine patients complained of pain averaging 5.3 VAS points (40.9%). The mean duration of treatment with Tegaderm™ Matrix was 9.4 weeks. Finally, the data records of 19 patients could be evaluated. The wound area of the patients at the initial visit was 0.1 cm2 to 23.6 cm2 on average 4.6 cm2 (median 1.9 cm2 ). At the follow up visit, the mean wound size had decreased to 3.8 cm2 (median 0.8 cm2 ) and at the final visit to 2.8 cm2 (median 0.5 cm2 ). There was at least a 50% reduction in wound area for 63.2% of the patients.
Discussion
In the context of a complex, causal treatment strategy for patients with chronic wounds, the use of modern
wound dressings makes sense, for example, for the realization of a moist wound environment as part of exudate management. It is propagated that in the case of therapy-refractory courses, the choice of a wound dressing can potentially re-induce the previously stagnant wound healing [8]. The studies available to date show that Tegaderm™ Matrix can have a positive effect on previously stagnant wound healing [9]. This is mainly attributed to the reduction in the pH value at the wound surface, which among other things contributes to reduced activity of the matrix metalloproteinases (MMPs) [9–11].
Clinical studies on Tegaderm™ Matrix
The current literature contains several publications on the clinical use of Tegaderm™ Matrix or the identical product DerMax®. Thus, Hamptom et al. in a prospective case study the positive influence of Tegaderm™ Matrix in 23 patients with chronic, non-healing wounds. Overall, complete healing was achieved in 48% of the patients [12]. Karim et al. described the wound healing processes of 4 patients with chronic wounds treated with Tegaderm™ Matrix. In this study, on day 0 and every 14 days thereafter
a biopsy was taken from the center of the wound until the wounds were completely healed. The histological examinations initially showed a high expression of MMP-2. After 14 days, there was a clinical reduction in the exudate, but
hardly any changes in the immunohistochemically examined patterns. After 4 weeks, in addition to a reduction in area, the beginning of epithelization and a further reduction in wound exudate could be described. In addition, there was now also a reduction in MMP-2 expression, which continued into week 6. The authors discussed that, among other things, the reduced MMP-2 expression readjusted the impaired wound healing and that the extent of the MMP-2 reduction under therapy with Tegaderm™ Matrix can be seen as a marker for the onset of wound healing [13]. Lassance et al. were able to show in biopsies that the previously increased MMP generation normalized during therapy with Tegaderm™ Matrix [14].
Two other clinical studies on patients with chronic wounds also showed good therapeutic success during treatment with Tegaderm™ Matrix. Thus, Pirayesh et al. a total of 20 patients with a previously therapy-refractory diabetic foot syndrome and a wound size of more than 2 cm2 over a total of 4 months. Complete wound closure occurred in 75% of patients within the observation period [15]. Also van Leen et al. treated 19 patients with decubitus ulcers of stages II–IV according to Seiler for a maximum of 6 weeks. All stage II and III patients healed completely. In the group of stage IV patients, healing was seen in only 20% [16]. In a prospective, open study by our working group, a total of 5 patients with chronic wounds that were extremely refractory to therapy were included. In addition to the clinical wound assessment, the pH value of the wound surface and the pain were also measured regularly. All patients showed a reduction in absolute wound size; one patient had a complete healing of the wound. The mean wound size was reduced from 13.5 cm2 to 3.1 cm2 .
The average pain intensity of the wounds decreased during therapy from 1.4 before therapy to 0.62 during therapy and 0.4 at the end of the observation period. In the course of our investigation, we were also able to objectify a previously undescribed significant decrease in the pH value in the wound environment [9].
MMPs and wound healing the exact interaction of the MMPs in vivo, which were first described in 1962, is still not fully understood. A central task of the group of MMPs is the homeostasis of the extracellular matrix (ECM) and the basement membrane zone [17]. Thus, MMPs promote the breakdown of damaged cell material, cellular migration in the context of re-epithelialization, and enable an optimization of tissue remodeling after wound closure [18]. Impaired control of the interaction of MMPs and TIMPs leads to increased damage to the ECM with successive un-physiological tissue destruction. The result is impaired wound healing as part of a perpetual inflammatory reaction. In addition, other building blocks that are essential for undisturbed wound healing, such as various growth factors, can be degraded by MMPs. It has already been shown that in this complex interplay of different enzymes such as MMP-2, among other things, are jointly responsible for impaired re-epithelialization due to increased degradation of collagen [19, 20].
The level of MMP-2 in exudate from chronic wounds is significantly increased compared to acute wounds [21, 22]. Initial clinical studies have shown that the use of modern wound dressings leads to a significant reduction in MMP-2 in wound exudate. These MMP-2 decreases correlate directly with the reduction in wound area or with a promotion of neoangiogenesis and re-epithelialization [20, 23]. A modulation of the proinflammatory, catabolic wound environment, for example through the use of Tegaderm™ Matrix, can therefore be a factor in inducing wound healing, particularly in wounds that are refractory to therapy.
PHI-5
Under the name PHI-5, various metal ions are used in combination with citrate buffer as the active components of Tegaderm™ Matrix. Using PHI-5, a reduction of both reactive oxygen species (ROS) and MMP-2 could be shown [11, 23]. An increased occurrence of these catabolic components of the wound environment otherwise leads to a forced breakdown of growth factors and protease inhibitors. The resulting oxidative stress can also promote perpetual inflammation and lead to an imbalance between tissue formation and breakdown in chronic wounds [3]. In vitro studies show that both a reduction in the release of ROS from polymorphonuclear neutrophils (PMN) and an inhibition of complement activation can be achieved by PHI-5. Through the indirect inhibition of ROS and the reduced complement activation by PHI-5, this tissue-destructive cascade can be interrupted and protection of the tissue can be achieved [9].
A study on guinea pigs showed that PHI-5 has a positive effect on the healing of acute wounds. In this work, except in the control group, an increasing concentration of PHI-5 in the wounds was used. The differentiated analysis showed a significantly reduced wound size in the group treated with higher PHI-5 concentrations after one week [24]. In none of the studies published to date has there been any indication of cytotoxicity of the concentrations of PHI-5 used.
pH regulation The activity of MMPs is directly dependent on the pH of the surrounding environment. The activity optimum should be in the alkaline range between pH 7 and 8. In our own clinical studies, we were able to show that even chronic wounds usually have a rather alkaline pH value of around 7.4 [10].
In further studies it could then be shown that by lowering the pH value by one log level, a reduction in the activity of various proteolytic enzymes in chronic wounds and in particular for MMP-2 of more than 80% can be achieved
[5]. Since Tegaderm™ Matrix contains a citrate buffer system and the lowering of the pH value on the wound surface has already been proven [9], the wound healing-promoting effect can be discussed through a reduction or normalization of MMP activity and increased oxygen uptake.
Own data
The “Patient Outcome Group” of the Community of European Wound Healing Societies (EMWA) pointed out in a consensus statement in 2010 that in clinical studies on patients with chronic wounds a reduction in the wound area of at least 50% is a parameter for successful treatment should be taken as a basis [25]. In this respect, in the following we have placed particular value on this aspect, which is very relevant in practice.
In the total group of 364 patients included in the study, the progression of the wound surfaces in 314 patients could be fully documented and evaluated. In 72.9% of these patients with previously refractory wounds, treatment with Tegaderm™ Matrix achieved a wound area reduction of at least 50%. A total of 14.3% of the documented wounds healed completely.
Subgroup analyzes were performed for the three most common entities of a chronic wound – leg ulcers, diabetic foot ulcers and decubitus ulcers. Of the 231 patients with a leg ulcer, the wound surfaces were fully documented for 207 patients and could be evaluated for the healing rate. In 69.1% of these patients, the treatment achieved a wound area reduction of at least 50%.
A total of 12.1% of the documented patients healed completely. Of the 9 patients with a decubitus ulcer, the wound area was fully documented in 8 patients for all three visits. At least a 50% reduction in wound area was achieved for 5 of the 8 patients (62.5%). One patient’s wound healed completely within the specified study period. In the 22 patients with a diabetic foot syndrome, the healing rate could be evaluated for 19 patients. A total of 63.2% of the patients showed a wound area reduction of at least
50% under treatment. Complete wound closure was achieved in 21.1% of patients. By specifying that in the context of this study, the accompanying causal therapeutic measures already introduced, such as compression therapy in patients with a venous leg ulcer or pressure relief in patients with decubitus ulcers, should be continued unchanged, the conclusion that the objectified effects could essentially be achieved through the use of Tegaderm™ Matrix suggests itself. However, since there is no control group in the study design, the influence of other factors cannot be ruled out. Further options of modern wound therapy are available for patients in whom complete wound closure could not be achieved [26, 27].
Based on our results, it is not intended to propagate that Tegaderm™ Matrix is now the wound dressing to be used in all patients with a chronic wound in the future. In every patient with a chronic wound, the primary aim should be to consistently implement a causal treatment concept, such as phlebosurgical intervention and compression therapy in patients with a venous leg ulcer. However, if wound healing stagnates despite treatment of the underlying factors accompanied by modern moist wound therapy, then this can be activated in at least some of the patients by using Tegaderm™ Matrix, for example.
So far, however, there have been nosuitable test systems that would enable suitable patients to be selected in advance. According to the current announcements at scientific congresses, these test systems, which would enable individualized therapy, can be expected in the near future.
Conclusion
Integrated into a causal therapy, Tegaderm™ Matrix is a well-tolerated wound dressing for many patients with previously therapy-refractory chronic wounds. Activation of the wound healing process
in more than 70% of patients supports.
Conflict of interest
Statement: This clinical study was commissioned by the company 3M (Neuss). The authors Körber and
Dissemond have held paid lectures for 3M on several occasions in recent years. Prof. Dissemond has also served as Scientific Advisor and Director for several 3M clinical trial projects.
Correspondence address
Prof. Dr. medical Joachim Dissemond Clinic and Polyclinic for Dermatology, Venereology and Allergology Essen University Hospital Hufelandstraße
55 D-45122 Essen Tel.:
+49-201-723-3894 Fax:
+49-201-723-5935
E-mail: joachimdissemond@hotmail.com
Literature
1 Dissemond J, Körber A, Grabbe S.
Differential diagnoses of leg ulcers.
J Dtsch Dermatol Ges 2006; 8:627-34.
2 Körber A, Klode J, Al-Benna S, Wax C,
Schadendorf D, Steinsträsser L,
Dissemond J. Genesis of chronic leg
ulcers in 31,619 patients as part of an
expert survey in Germany. J Dtsch
Dermatol Ges 2011; 9:116-22.
3 Dissemond J, Goos M, Wagner SN.
The role of oxidative stress in the Pathogenesis and therapy of chronic wounds. Dermatologist 2002; 53:718-23.
4 Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wo und healing. Int J Biochem Cell Biol 2008; 40: 1334–47.
5 Greener B, Hughes AA, Bannister NP, Douglass J. Proteases and pH in chronic wounds. J Wound Care 2005; 14: 59–61.
6 Schneider LA, Körber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res 2006; 298: 413–20.
7 Goldman RJ, Salcido R. More than one way to measure a wound: An overview of tools and techniques. Adv Skin Wound Care 2002; 15: 236–43.
8 Körber A, Weindorf M, Dissemond J. Exudate management capacity of modern wound dressings for the therapy of Ulcus cruris venosum under compression therapy. Dermatologist 2008; 59:904-11.
9 Körber A, Freise J, Rietkötter J, Grabbe S, Dissemond J. Successful treatment of refractory chronic wounds with DerMax. ZfW 2006; 11:310-4.
10 Dissemond J, Witthoff M, Brauns TC, Haberer D, Goos M. Investigations on the pH value of the milieu of chronic wounds in the context of a modern wound therapy. Hautarzt 2003; 54: 959–65.
11 van den Berg AJ, Halkes SB, van Ufford HC, Hoekstra MJ, Beukelman CJ. A novel formulation of metal ions and
citric acid reduces reactive oxygen species in vitro. J Wound Care 2003; 12: 413–8.
12 Hampton S, Young S, Kerr A, King L. An observational study of the use of a ionogen impregnated dressing (DerMax) in the treatment of wounds. Poster shown at EWMA-Con gress 2006, Prag.
13 Karim RB, Brito BL, Dutrieux RP, Lassance FP, Hage JJ. MMP-2 assessment as an indicator of wound healing: A feasibility study. Adv Skin Wound Care 2006; 19: 324–7.
14 Lassance FP, Karim RB, Brito B, Raaij maker J, Joris Hage J. Analysis of morphological and immunhistological changes during treatment with Der Max. Wound Repair Regen 2003; 11: 53.
15 Pirayesh A, Dessy LA, Rogge FJ, Hoeksema HJ, Sinove YM, Dall’ Antonia A, Jawad MA, Gilbert PM, Rubino C, Scuderi N, Blondeel R, Monstrey S. The efficacy of a polyhydrated ionogen impregnated dressing in the treatment of recalcitrant diabetic foot ulcers: a multi-center pilot study. Acta Chir Belg 2007; 107: 675–81.
16 van Leen MWF, Lüning EWN, Neyens
JCL, Schols JMGA. Naaldwijk. Analysis of morphological and immunohistochemical changes during treatment with Dermax®. WUWHS 2004, Paris.
17 Wang X, Li KF, Adams E, van Schepdael A. Matrix metalloproteinase inhibitors: a review on bioanalytical methods, pharmacokinetics and meta bolism. Curr Drug Metab 2011; 12: 395–410.
18 Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteina
ses and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetology 2002; 45:1011–6.
19 Mwaura B , Mahendran B , Hynes N , Defreitas D , Avalos G , Adegbola T , Adham M , Connolly CE , Sultan S . The impact of differential expression of extracellular matrix metallopro teinase inducer, matrix metallopro teinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg 2006; 31: 306–10.
20 Xue M, Le NT, Jackson CJ. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets 2006; 10: 143–55. 21 Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen 2009; 17: 832–9.
21 Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous legulcers. Wound Repair Regen 2009; 17:832–9.
22 Wiegand C, Schönfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are chronically elevated compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res 2010; 302: 419– 28.
23 Motzkau M, Tautenhahn J, Lehnert H, Lobmann R. Expression of matrix-me talloproteases in the fluid of chronic diabetic foot wounds treated with a protease absorbent dressing. Exp Clin Endocrinol Diabetes 2011; 119: 286–90.
24 van Rossum M, Vooijs DPP, Walboo mers XF, Hoeckstra MJ, Spauwen PHM, Jansen JA. The influence of a PHI-5- loaded silicone membrane, on cutaneous wound healing in vivo. J Mater Sci Mater Med 2 007; 18: 1 449– 5 6.
25 Gottrup F, Apelqvist J, Price P, EWMA Patient Outcome Group. Outcomes in controlled and comparative studies on non-healing wounds: Recommendations to improve the quality of evidence in wound management. J Wound Care 2010; 19: 237–68.
26 Dissemond J. Wound management – State of the Art 2011. Compendium Dermatology 2011; 7: 26–9.
27 Dissemond J. Physical therapies of chronic leg ulcers. dermatologist 2010; 61: 387–96.


